Impact That Matters
Therapeutic Research In Accelerated Discovery (TRIAD)TRIAD = Academia + Government + Industry
TRIAD Partners
Our TRIAD partners include innovative biotech & pharmaceutical companies, award-winning researchers at top-tier universities, and those spanning the drug discovery, drug development, and diagnostics continuum for Charcot-Marie-Tooth research.
Partnerships vary depending on research goals, milestones met in preclinical or clinical development, and strategic alliance agreements.
TRIAD & GRIN
As part of TRIAD, in 2013, the Global Registry for Inherited Neuropathies (GRIN) was developed to conduct patient-focused research & development for treatments and cures. The patient voice is at the forefront of all we do. By incorporating the patient voice from the beginning, our research programs have the greatest potential for success. HNF funds research with one goal in mind; advancing to clinical trials for Charcot-Marie-Tooth research.
TRIAD Council
The TRIAD Council is composed of CMT thought leaders, experts, and consultants engaged in collaborative planning and decision-making to provide regular guidance and direction to our research strategy. This network of professionals reviews grant proposals, provides expert guidance, and assesses project outcomes to advance therapeutic development for CMT.
Our most recent TRIAD partnerships & initiatives
Click on the logos for more information.
Click on the logos for more information.





HNF participates and supports an advocacy role by providing real-world data to the FDA and other stakeholders. Our critical data facilitates improved knowledge of the lived experience of CMT patients and the advancement of clinical development.
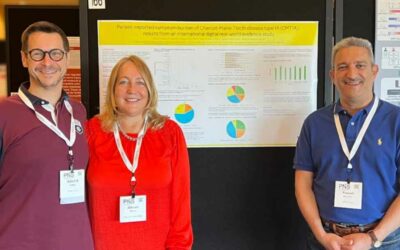
CMT&ME App Study Presented at PNS Meeting 2022
The CMT&Me real-world digital lifestyle study self-reports data from patients with all types of CMT and is collected on a regular basis in both US and Europe.
Pharnext Announces On-Schedule Completion of Patient Enrollment in its Pivotal Phase III Trial of PXT3003, the PREMIER Trial, for the Treatment of Charcot-Marie-Tooth Disease Type 1A (CMT1A)
Pharnext Announces On-Schedule Completion of Patient Enrollment in its Pivotal Phase III Trial of PXT3003, the PREMIER Trial, for the Treatment of Charcot-Marie-Tooth Disease Type 1A (CMT1A)
3 New CMT Drug Development Projects
HNF Announces 3 New Drug Partnerships & Pathways Targeting Multiple Forms of CMT.
What is SORD Deficiency: Part 2
Join us for this webinar as we learn more about Sorbitol Dehydrogenase (SORD) Deficiency
A study of the correlation between gait abnormalities, activity monitoring parameters, CMTPedS and a biomarker in children with Charcot-Marie-Tooth disease.
A study of the correlation between gait abnormalities, activity monitoring parameters, CMTPedS and a biomarker in children with Charcot-Marie-Tooth disease.
Applied Therapeutics Announces Initiation of Registrational Phase 2/3 Study of AT-007 in SORD Deficiency
Press Release
Applied Therapeutics Reports Biomarker Data from Pilot Trial of AT-007 in SORD Deficiency
Press release
HNF collaborates with Rarebase on a Drug Discovery Platform to develop treatments for Charcot-Marie-Tooth (CMT)
HNF collaborates with Rarebase on a Drug Discovery Platform to develop treatments for Charcot-Marie-Tooth (CMT)
New Study Measures Progression of CMT1A Nerve Impairment
New Study Measures Progression of CMT1A Nerve Impairment
Interim Analysis Shows Sustained Benefits of PXT3003 for Patients with Charcot-Marie-Tooth Disease Type 1A (‘CMT1A’)
Study Shows Sustained Benefits of PXT3003 for Patients with Charcot-Marie-Tooth Disease Type 1A (‘CMT1A’)