Impact That Matters
Learn about HNF’s Research InitiativesResearch programs that can change people’s lives
We prioritize research programs that will change people’s lives by partnering with innovative researchers and industry leaders.
We ONLY support projects that have potential to have an impact on human health.
- To partner, contact Joy Aldrich: [email protected]
- To submit a research proposal, contact Allison Moore: [email protected]
TRIAD
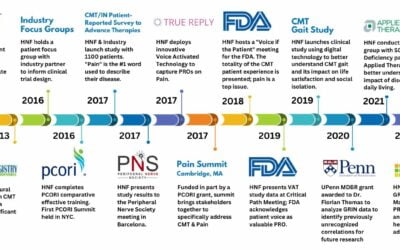
HNF developed the Therapeutic Research in Accelerated Discovery (TRIAD) as a patient-led collaborative network with academia, government, and industry to develop treatments for CMT. Currently TRIAD involves many groups that span the drug discovery, drug development, and diagnostics continuum.
Our core philosophy mandates that we collaborate with our patient community and strategic partners compassionately, honestly and with integrity. The patient always comes first and drives our patient support and research efforts.
CMT & Pediatrics
HNF has a dedicated CMT & Pediatric initiative to support the development of clinical trials.
GRIN
As part of TRIAD, in 2013, the Global Registry for Inherited Neuropathies (GRIN) was developed as an extension to conduct patient-focused research & development for treatments and cures. The patient voice is at the forefront of all we do. By Incorporating the patient voice from the very beginning, our research programs have the greatest potential for success.
HNF funds research with one goal in mind, advancing to clinical trials. Partnerships vary depending on research goals, milestones met, preclinical or clinical development and the strategic alliance agreement.
Good News for CMT1A Patients – PXT3003
Three major regulatory agencies in the United Kingdom, Europe and US have recognized PXT3003 as a lead drug candidate to treat CMT1A.
Novel findings of a new common type of CMT2 that might be the 1st step to a treatment: SORD gene deficiency, the most common autosomal-recessive type of CMT
a mutation in the SORD gene that may may affect 60,000 patients worldwide
GRIN powers HNF’s patient-centered research and drug discovery initiatives.
GRIN has played a critical role in identifying important issues related to the CMT patient experience, revealing new areas to explore and research.
$61,000 Raised to Support Pediatric CMT Trials
Last summer HNF teamed up with the Penn Medicine Orphan Disease Center for the Million Dollar Bike Ride in Philadelphia.
Pharnext Announces PXT3003 for the Treatment of Charcot-Marie-Tooth Disease Type 1A has Been Granted Promising Innovative Medicine (PIM) Designation by UK Medicines and Healthcare Products Regulatory Agency
United Kingdom’s Medicine and Healthcare products Regulatory Agency (MHRA) has granted Promising Innovative Medicine (PIM) designation to its lead drug candidate, PXT3003, for the treatment of Charcot-Marie-Tooth Disease Type 1A (CMT1A) in patients 16 years and older.
Pharnext raises € 7.7 million in a private placement
Pharnext SA (FR0011191287 – ALPHA) (the “Company”), a biopharmaceutical company pioneering a new approach to developing innovative drug combinations based on its PLEOTHERAPY artificial intelligence platform harnessing big genomics data and network pharmacology, today announced a capital raise of circa € 7.7 million by way of issuance of 1,799,061 new ordinary shares (the “New Shares”) with one warrant attached each (together with the New Shares, the “ABSA”).
HNF on the Forefront of Pain Research to Support CMT Patients
Allison Moore, HNF Founder/CEO, along with her team, took action and led the HNF groundbreaking CMT pain initiative to help the community.
EmBRACE It Podcast with Lainie Ishbia and Estela Lugo
We want our CMT viewers and listeners to feel like they can relate to us, and that it’s perfectly okay to be imperfect!
Hope for the Future for Owen
A gene therapy is within our reach for GDAP1 (CMT4A), an autosomal recessive loss of function disorder and effecting many patients like Owen.
CMT&ME APP Adds New Medical Profile
The CMT&ME app has updated the research app to include a brand new medical profile.