Impact That Matters
Learn about HNF’s Research InitiativesResearch programs that can change people’s lives
We prioritize research programs that will change people’s lives by partnering with innovative researchers and industry leaders.
We ONLY support projects that have potential to have an impact on human health.
- To partner, contact Joy Aldrich: [email protected]
- To submit a research proposal, contact Allison Moore: [email protected]
TRIAD
HNF developed the Therapeutic Research in Accelerated Discovery (TRIAD) as a patient-led collaborative network with academia, government, and industry to develop treatments for CMT. Currently TRIAD involves many groups that span the drug discovery, drug development, and diagnostics continuum.
Our core philosophy mandates that we collaborate with our patient community and strategic partners compassionately, honestly and with integrity. The patient always comes first and drives our patient support and research efforts.
CMT & Pediatrics
HNF has a dedicated CMT & Pediatric initiative to support the development of clinical trials.
GRIN
As part of TRIAD, in 2013, the Global Registry for Inherited Neuropathies (GRIN) was developed as an extension to conduct patient-focused research & development for treatments and cures. The patient voice is at the forefront of all we do. By Incorporating the patient voice from the very beginning, our research programs have the greatest potential for success.
HNF funds research with one goal in mind, advancing to clinical trials. Partnerships vary depending on research goals, milestones met, preclinical or clinical development and the strategic alliance agreement.
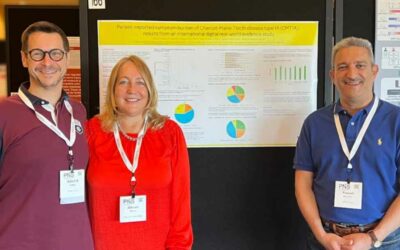
CMT&ME App Study Presented at PNS Meeting 2022
The CMT&Me real-world digital lifestyle study self-reports data from patients with all types of CMT and is collected on a regular basis in both US and Europe.
Pharnext Announces On-Schedule Completion of Patient Enrollment in its Pivotal Phase III Trial of PXT3003, the PREMIER Trial, for the Treatment of Charcot-Marie-Tooth Disease Type 1A (CMT1A)
Pharnext Announces On-Schedule Completion of Patient Enrollment in its Pivotal Phase III Trial of PXT3003, the PREMIER Trial, for the Treatment of Charcot-Marie-Tooth Disease Type 1A (CMT1A)
3 New CMT Drug Development Projects
HNF Announces 3 New Drug Partnerships & Pathways Targeting Multiple Forms of CMT.
What is SORD Deficiency: Part 2
Join us for this webinar as we learn more about Sorbitol Dehydrogenase (SORD) Deficiency
A study of the correlation between gait abnormalities, activity monitoring parameters, CMTPedS and a biomarker in children with Charcot-Marie-Tooth disease.
A study of the correlation between gait abnormalities, activity monitoring parameters, CMTPedS and a biomarker in children with Charcot-Marie-Tooth disease.
Applied Therapeutics Announces Initiation of Registrational Phase 2/3 Study of AT-007 in SORD Deficiency
Press Release
Applied Therapeutics Reports Biomarker Data from Pilot Trial of AT-007 in SORD Deficiency
Press release
HNF collaborates with Rarebase on a Drug Discovery Platform to develop treatments for Charcot-Marie-Tooth (CMT)
HNF collaborates with Rarebase on a Drug Discovery Platform to develop treatments for Charcot-Marie-Tooth (CMT)
New Study Measures Progression of CMT1A Nerve Impairment
New Study Measures Progression of CMT1A Nerve Impairment
Interim Analysis Shows Sustained Benefits of PXT3003 for Patients with Charcot-Marie-Tooth Disease Type 1A (‘CMT1A’)
Study Shows Sustained Benefits of PXT3003 for Patients with Charcot-Marie-Tooth Disease Type 1A (‘CMT1A’)